
Presently, the biopharmaceutical sector has emerged as the cornerstone of global pharmaceutical advancements. Its market share continues to rise annually, establishing itself as a pivotal force propelling the industry forward. This growth is forging new pathways and presenting more possibilities for the treatment of human diseases. However, the extended timeline of drug development and transformation remains a major hurdle for the biopharmaceutical industry. Effectively shortening this timeline and enhancing research and development efficiency are crucial challenges facing the sector.
All-in-one solution of Morimatsu tackles these challenges by encompassing upstream culture, downstream isolation and purification, and single-use systems for various application scenarios.
Upstream Culture Solution
MOCELLULA™ GBR/GFE Glass Bioreactors

✔ Flexible formulations for cell and microbial cultures
✔ Offer a range of volume specifications and flexible combinations in single, double, or multiple forms, tailored to highly customized needs
✔ The interactive interface is user-friendly, ensuring data integrity and traceability while enhancing stability and reliability
✔ Ergonomic design significantly enhances the operational experience
MOCELLULA™ WAV Wave Bioreactor

✔ Ideal for laboratory-scale cell culture, process development, scale-up, and cGMP commercial-scale production
✔ Offers multiple processes: suspension culture, perfusion culture, and microcarrier culture
✔ Multiple control modes: CO2 mixing control or pH/DO online control
✔ Management system includes integrity checking for precise control and tracking of culture parameters
MOCELLULA™ WAV-2D Single-use Bioreactor

✔ The unique two-dimensional movement pattern offers a low shear environment while showcasing outstanding oxygenation capacity with Kla up to 400h⁻¹
✔ Different series of specifications are available according to the varying needs of seed expansion and final product production for different cell types, such as CHO, HEK293, and Vero
✔ Uses a disposable containment system to significantly reduce the risk of contamination
✔ Employs a powerful control software system for precise control of key process parameters and data tracking, highly scalable
✔ Provides an all-in-one platform culture solution for cell therapy from R&D to commercialization
Downstream Separation and Purification Solution
MOCHROMA™ QBE Benchtop Chromatography Systems

✔ Ideal for the rapid purification of target products such as proteins, peptides, and nucleic acids ranging from micrograms to tens of grams
✔ Supports affinity, ion exchange, hydrophobic, molecular sieve, reversed-phase, multi-mode, and other mainstream chromatography technologies
✔ Incorporates imported key components for high precision and stability
✔ Control software integrates system control, method editing, data analysis, user rights management, audit trail, and other functions to ensure data security and integrity
✔ Provides high and low preparation options for simplified selection
MOPROCESS™SUI Single-Use Online Liquid Preparation/Cracking System

✔ Meets IC/ID/LS process needs and adapts to various production scales
✔ User-friendly human-computer interaction
✔ Modular all-in-one design
✔ High precision system dispensing
MOPURITY™SUF Single-Use Tangential Flow Filtration System

✔ Compatible with TFF/DF/VF/SPTFF processes
✔ Fully automatic operation for simplicity
✔ Small circulation volume
✔ Compact size
✔ Real-time monitoring and secure data storage of key parameters
Single-Use Solution
MOTRANSIT™ MIX/STO Single-Use Liquid Distribution and Storage System

✔ Moveable universal drive, with a wide range of Holder application volumes from 50L to 3000L
✔ Powerful mixing performance to accommodate solid-liquid and liquid-liquid mixing application conditions
✔ Disposable products are manufactured in a C+A environment, with accessories pre-cleaned for enhanced product cleanliness
✔ Flexible product configurations and customized services available
✔ Ergonomically designed overall structure for simplified operation
MOCELLULA™SUB Single-use Bioreactor

✔ Complete range of bioreactors from 50L to 2000L, offering comprehensive process scale-up solutions
✔ Advanced automation and modular system design, supporting various installation options
✔ No requirement for system cleaning and sterilization for validation, enabling multi-species co-cultivation
✔ Flexible product configurations and customized services are available
Single Use System
Morimatsu also offers single-use systems, including disposable 2D reservoir bags, disposable 3D reservoir bags, disposable sampling bags, disposable mixing bags, and customized disposable connectors. These systems come in a variety of specifications suitable for various equipment brands, ensuring a stable process, safe and reliable quality, and ample validation documentation to support them.
Application Scenario: Antibody Production Process
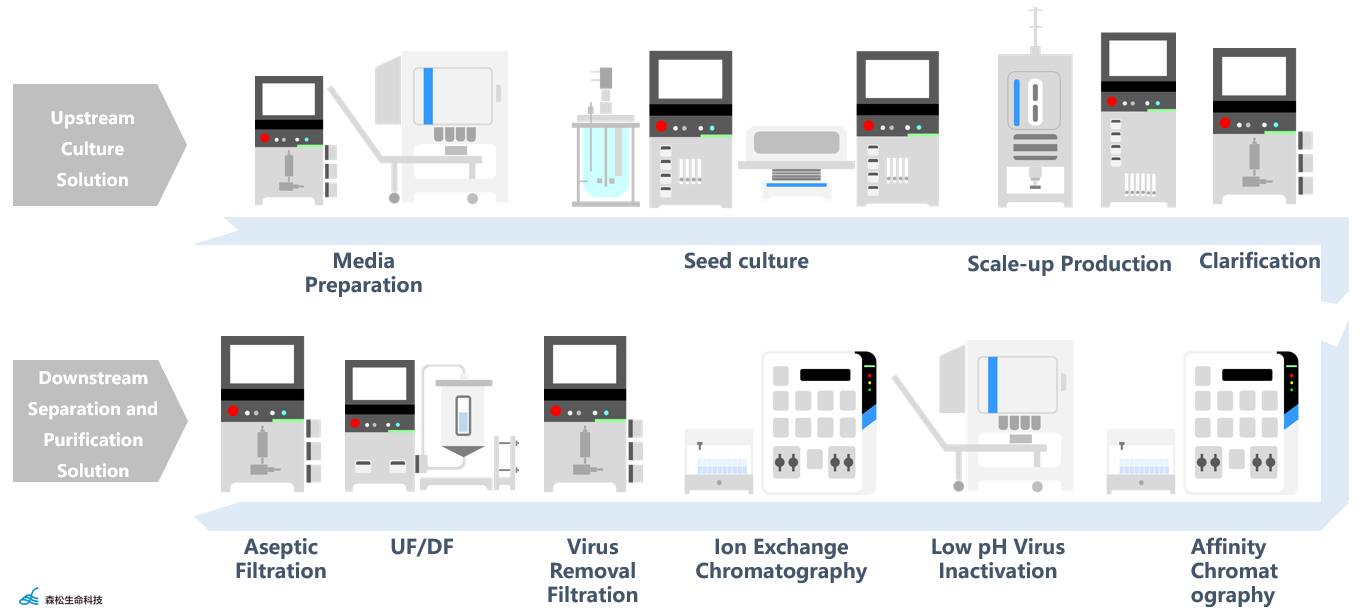
In the booming global biopharmaceutical industry, Morimatsu prioritizes customer needs, innovation, and strong support for research and production. This commitment aims to deliver greater value and propel ongoing advancements in human health.
About Morimatsu LifeSciences
Morimatsu LifeSciences, one of the key business segments of Morimatsu International Holding Co., Ltd. (Morimatsu International, stock code: 2155.HK), mainly consists of Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd., Morimatsu (Suzhou) LifeSciences Company Limited, Shanghai Morimatsu Biotechnology Co., Ltd., Shanghai Mori-Biounion Technology Co., Ltd. , Pharmadule Morimatsu AB (Sweden) and its subsidiaries, which serves the pharmaceuticals, bio-pharmaceutical, cosmetic medicine, FMCG (cosmetics, baby, women & home Care, health care, fabric & home care, food, beverage, nutraceuticals) and other industries, providing customers with "core equipment+value-added services+digital intelligent overall factory solutions and services" ("MVP Solutions&Services"), focusing on core equipment, stainless steel process systems, disposable process systems, consumables, laboratory solutions, digital and modular factory solutions and services.
As a diversified multinational company, Morimatsu has opened subsidiaries or advanced manufacturing plants in China, Japan, Sweden, United States, India, Italy, Singapore, and has delivered different forms of products and services to more than 40 countries and regions so far, by its global footprint of an efficient and professional team.
Forward-Looking Statements
The information in this press release may include some forward-looking statements. Such statements are essentially susceptible to considerable risks and uncertainties. The use of "predicted", "believed", "forecast", "planned" and/or other similar words/phrases in all statements related to our company is to indicate that the statements are forward-looking ones. Our Company undertakes no obligation to constantly revise such predicted statements.
Forward-looking statements are based on our Company management's current perspectives, assumptions, expectations, estimations, predictions and understanding of future affairs at the time of the making of such statements. Such statements are not guarantees of future development and are susceptible to the impact of risks, uncertainties and other factors; some are beyond the control of our Company and unpredictable. Subject to the influence of future changes and development in our business, competition environment, political, economic, legal and social conditions, the actual outcomes may differ significantly from the information contained in the forward-looking statements.