
In biopharmaceutical manufacturing, every step—from gene sequence to final product—must work in sync. Yet many companies still rely on equipment from multiple vendors, assembling complex systems piece by piece. The result? Integration challenges, disconnected data, and difficult technology transfers. Even small gaps between stages can affect efficiency and product consistency.
There’s a better way to solve the puzzle. With integrated, end-to-end process equipment, operations that once ran in isolation can be connected into a streamlined, reliable production system. Morimatsu’s solutions are built around this approach, covering the critical stages of biopharmaceutical manufacturing and helping companies create a smooth, connected workflow—from DNA to final drug product.
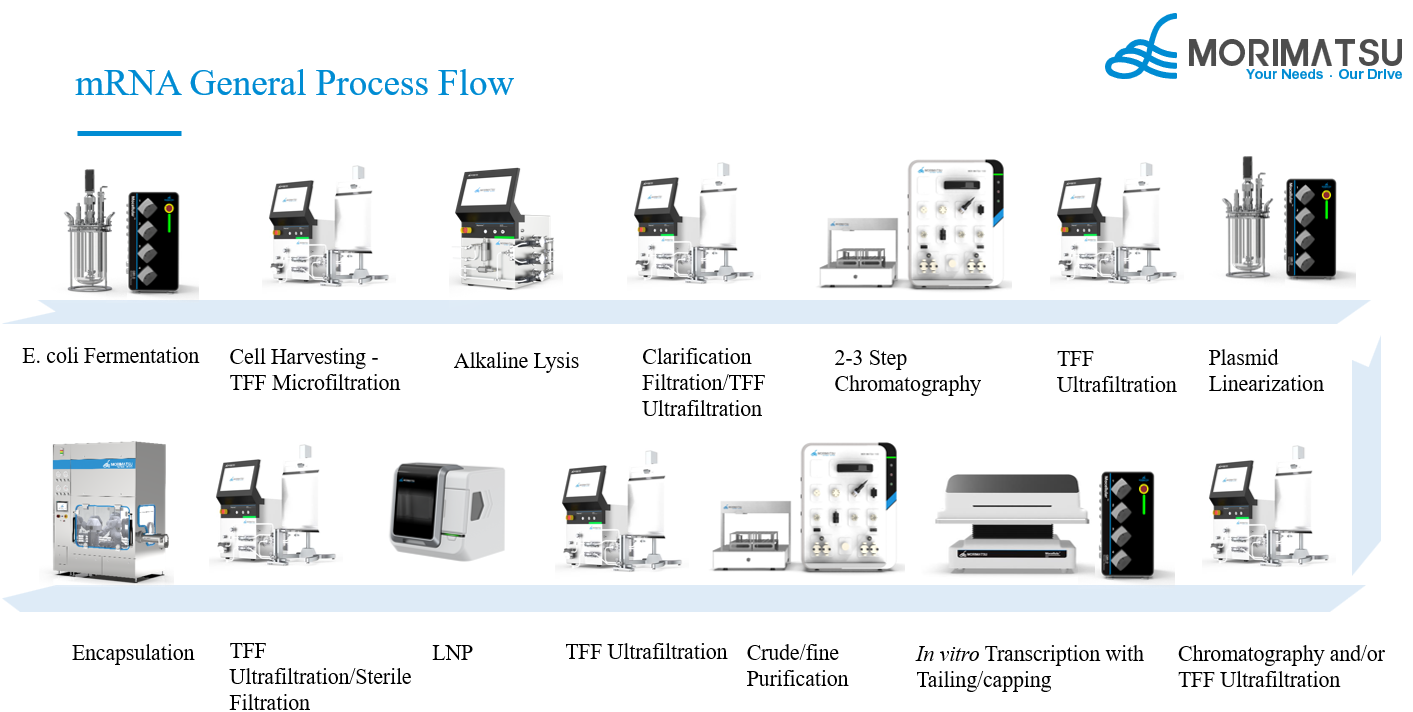
Figure 1 mRNA General Process Flow
The Foundation of High-Efficiency Expression
Every biopharmaceutical starts with efficient expression in genetically engineered cells. As a proven system for plasmid production, E. coli fermentation creates the conditions needed for strong cell growth and product formation. Morimatsu’s fermentation equipment supports high-density cultivation of engineered strains with precise control of temperature, pH, dissolved oxygen, and nutrient feeding—building a reliable foundation for downstream yield.

Figure 2 Morimatsu Glass Bioreactor
After fermentation, the next challenge is efficiently releasing intracellular products. Alkaline lysis uses carefully controlled conditions to gently break open cell membranes, releasing the target material while preserving its bioactivity. The effectiveness of this step has a direct impact on the starting quality of downstream purification.

Figure 3 Morimatsu Single-Use Lysis System
Downstream Purification—From Crude Extract to High-Purity Product
Once the cell lysate is obtained, the focus shifts to systematically removing impurities to isolate the target product. Clarification and ultrafiltration mark the first step in downstream purification, removing cell debris, host proteins, and other large contaminants while providing initial clarification and concentration of the process stream.

Figure 4 Morimatsu Clarification Filtration/Ultrafiltration System
Next come the capture and polishing steps. Using chromatographic separation, these stages selectively isolate the target product while removing host cell proteins, nucleic acids, endotoxins, and other impurities to achieve pharmaceutical-grade purity. For nucleic acid–based therapies, such as mRNA, in vitro transcription, capping, and linearization ensure template accuracy and RNA integrity, setting the stage for downstream use.

Figure 5 Morimatsu Benchtop Chromatography System
Ultrafiltration and diafiltration (UF/DF) play a key role during purification, enabling rapid buffer exchange, desalting, and formulation adjustments to place the product in its optimal environment. Consistent performance across the process depends on tight control and smooth integration of every unit operation.
Formulation and Filling—Final Product Formation and Safety Assurance
Purified drug substances must be carefully formulated to become stable, safe final products. For advanced therapies, such as LNP-based delivery systems, specialized preparation equipment uses microfluidic technology to precisely combine lipids with active ingredients, forming uniform, stable nanoparticles that protect the drug and enable efficient delivery.

Figure 6 Morimatsu Intelligent Microfluidic Synthesizer
During filling, TFF and sterile filtration provide a final safeguard, ensuring each dose meets sterility requirements. Filling systems then deliver accurate, high-speed dispensing in a controlled environment, maintaining dose precision and container integrity for vials, prefilled syringes, and other primary packaging—fully aligned with GMP expectations.
Beyond Standalone Equipment: The Value of Integration
With a deep understanding of the full biopharmaceutical workflow, Morimatsu delivers solutions that span upstream expression, downstream purification, and final formulation and filling.
1.Process Continuity
From development through commercial-scale production, each system is designed, validated, and optimized to support smooth scale-up and reduce risk during technology transfer.
2.Data Integrity
An integrated platform enables seamless data collection and monitoring, supporting compliance with data integrity standards and providing a solid foundation for traceability and process optimization.
3.Efficiency Improvement
By minimizing non-core efforts—like matching equipment from different vendors or repeated validations—teams can focus on the process itself, speeding up time-to-market.
4.Risk Control
Working with a single supplier clarifies responsibilities, reduces supply chain and technical coordination risks, and helps ensure project timelines and quality goals are met.
Technical Advantages: Expertise Driving End-to-End Solutions
Morimatsu's process equipment demonstrates technical strengths across multiple dimensions:
1.Equipment Design
Morimatsu’s modular approach combines standardization with reliability while allowing flexibility for client-specific needs. From lab-scale to commercial production, key parameters and operating logic stay consistent, minimizing technical risks during scale-up.
2.Control System
Morimatsu’s advanced automation platform allows real-time monitoring and precise control of critical process parameters— temperature, pressure, flow, and pH—ensuring consistent batch-to-batch performance. The system also supports data logging and traceability, meeting GMP standards and providing reliable quality assurance.
3.Material Selection
All product-contact components use pharmacopeia-compliant materials such as 316L stainless steel, PTFE, and EPDM, ensuring compatibility with pharmaceutical fluids and minimizing leachables. For sterile processes, equipment is designed for easy cleaning and sterilization, featuring CIP/SIP functionality to boost efficiency while maintaining product quality.
4.Application Examples
Morimatsu’s advanced, flexible process equipment has proven its value across multiple biopharmaceutical projects, particularly in plasmid DNA production. In one gene therapy and nucleic acid vaccine project, the client implemented Morimatsu’s integrated solution from the early stages of process development.
During upstream fermentation, Morimatsu’s high-density system and optimized control strategies enabled efficient expression of engineered bacteria. The fermentation cycle was maintained at 21 hours, with cell density (OD600) consistently around 60, resulting in a substantial increase in plasmid DNA yield.
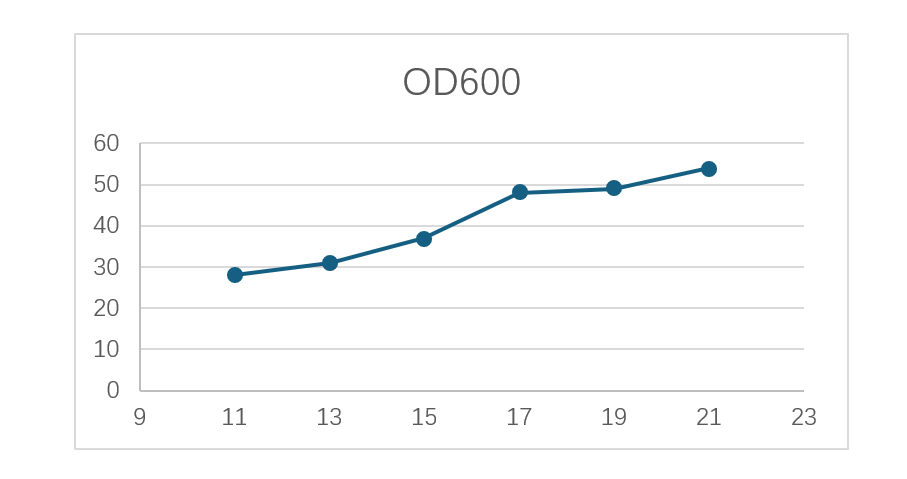
Figure 7 E. Coli Culture Curve
In another mRNA vaccine project, Morimatsu’s in vitro transcription and LNP preparation systems were key to success. The transcription reaction finished in just six hours, with mRNA capping efficiency above 95% and excellent molecular integrity. Using microfluidic technology, the LNP preparation achieved uniform particle size and over 90% encapsulation efficiency. From DNA template to LNP formulation, the fully integrated process supported continuous operation, significantly shortening the production cycle and enabling rapid vaccine development and manufacturing.
From raw materials to finished products, every step in biopharmaceutical production reflects the seamless integration of science, technology, and engineering. Morimatsu’s process equipment, built on advanced technology and guided by customer needs, helps bring innovative therapies to market efficiently and with consistent quality—delivering real benefits to patients around the world.
About Morimatsu LifeSciences
Morimatsu LifeSciences is a key business segment of Morimatsu International Holdings Limited (Stock Code: 2155.HK). It comprises Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd., Morimatsu (Suzhou) LifeSciences Co., Ltd., Shanghai Morimatsu Biotechnology Co., Ltd., Shanghai Mori-Biounion Technology Co., Ltd., Shanghai Morisora Technology Co., Ltd., Bioengineering AG, Pharmadule Morimatsu AB, and its affiliated companies.
Morimatsu LifeSciences is dedicated in providing core equipment, process systems, and smart modular facility solutions, and services for the pharmaceutical, biopharmaceutical, medical aesthetics, and fast-moving consumer goods (FMCG) sectors including (cosmetics, food, and health supplements), as well as data centers.
Our team comprises highly experienced professionals with deep expertise in process R&D, engineering design, advanced manufacturing, compliance and validation consulting, production execution, and intelligent operations. With broad experience across diverse industries, we fully understand the unique characteristics and process requirements of various products. This enables us to deliver tailored, end-to-end process solutions from the conceptual design stage, precisely aligned with client’s specific needs.
Morimatsu LifeSciences has established a strong global presence, supported by advanced R&D centers, design hubs, and state-of-the-art manufacturing facilities worldwide. Our well-established service network spans Europe,USA,Asia-Pacific, and emerging markets. We have successfully delivered outstanding, customized solutions to clients in over 40 countries and regions, gaining extensive experience in international project execution.
As a multinational enterprise with core strengths in process technology, modular facility construction, and intelligent manufacturing, Morimatsu LifeSciences is dedicated to meeting the evolving equipment and system needs of our key industries. Through continuous innovation and technological advancement, we are steadily expanding our global footprint, driving our international strategy forward, and delivering Morimatsu’s expertise, reliability, and innovation to the global life sciences and related sectors.