
As regulatory scrutiny tightens and market competition intensifies, traditional production management models in pharmaceutical manufacturing are showing clear limitations:
· Heavy reliance on paper-based records increases the risk of data errors, missing entries, and non-compliant signatures;
· Limited end-to-end traceability drives up compliance risk and audit pressure;
· Weak integration between equipment and production processes creates persistent data silos;
· Batch record review is slow and labor-intensive, with high error rates and long audit preparation cycles;
· High-risk steps such as weighing and batching remain vulnerable to material mix-ups, making effective risk control difficult.
Serving as the digital bridge between planning and shop-floor execution, the Manufacturing Execution System (MES) replaces paper batch records with Electronic Batch Records (EBR) through real-time production monitoring, automated data capture, and end-to-end audit trails. This enables a fundamental shift from manual processes to automation, and from fragmented data to centralized, traceable records. The result is a complete, reliable data backbone that spans from formulation through finished product—supporting lean manufacturing, regulatory compliance, and full lifecycle traceability.
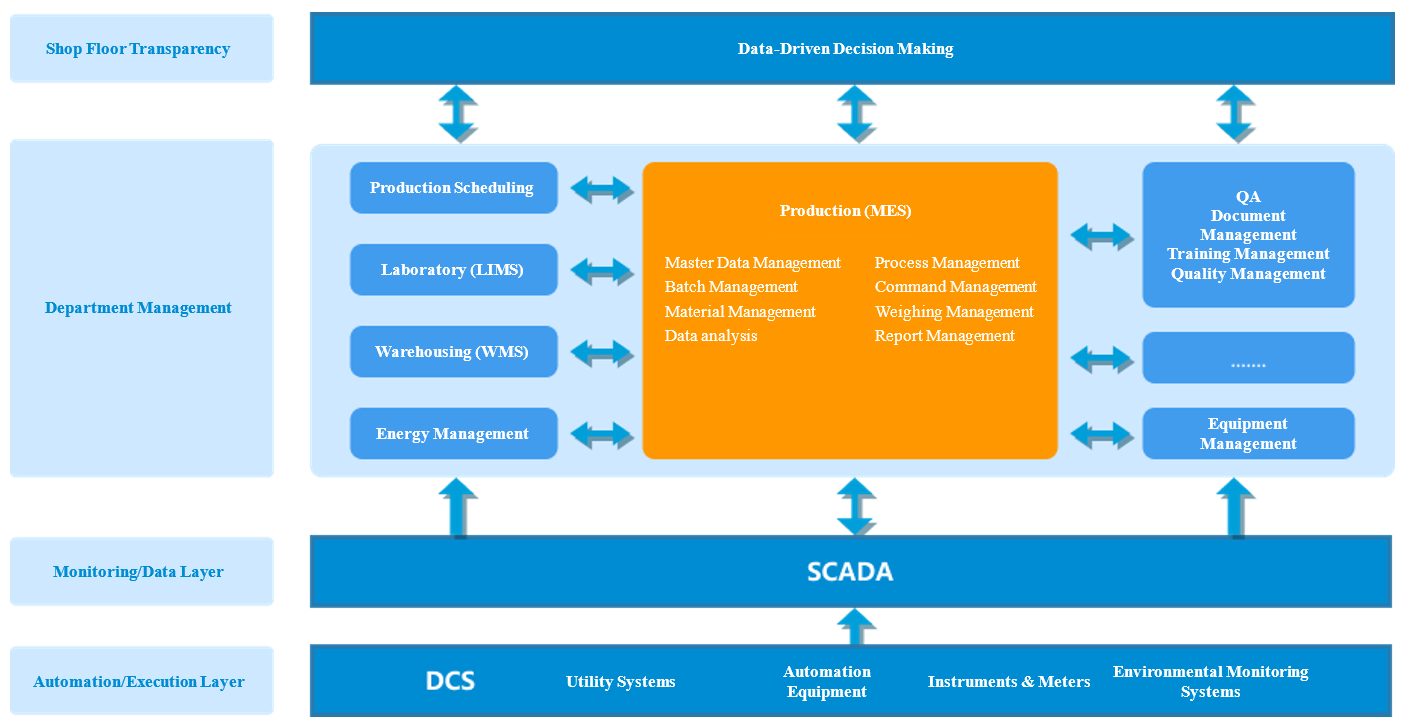
MES System Functional Architecture
Morimatsu MES: Nine Core Modules
01 Production Management: Manages production scheduling, material movement, and inventory control to ensure accurate execution of production plans while supporting end-to-end audit traceability.
02 Data Management: Centralizes master data—including work centers, equipment, materials, and batch inventory—to create a standardized, reliable data foundation that supports informed production decisions.
03 Recipe Management: Manages the full recipe lifecycle with precise parameter control, standardizing processes to ensure consistent production performance and reduce deviation risk at the source.
04 Production Execution: Covers end-to-end execution, including work order processing, material issuance and returns, and equipment cleaning. Enables closed-loop process control and real-time inventory synchronization across production.
05 Production Anomaly Management: Allows rapid capture of production exceptions, intelligent batch record review, and streamlined investigation and closure. Significantly shortens batch release timelines while maintaining a strong balance between compliance and operational efficiency.
06 Equipment Management: Provides real-time visibility into equipment status, qualification, and usage history. Shifts maintenance from reactive fixes to predictive strategies, reducing unplanned downtime and ensuring production continuity. Also manages scale status, calibration testing, and equipment labeling to support compliance and accuracy.
07 Weighing & Batching: Delivers precise batching control through real-time weight verification, digital records, and automated data capture. Barcode scanning validates material identity and key attributes at the point of use, preventing mix-ups and eliminating errors at the source.
08 System Integration: Seamlessly integrates with WMS, EAM, and other enterprise systems, as well as electronic regulatory platforms. Breaks down data silos and enables smooth, end-to-end data flow across systems.
09 Report Management: Automatically generates production compliance and inventory analysis reports. Supports fast label printing and one-click inventory inquiries, making data traceability efficient, transparent, and easy to access.
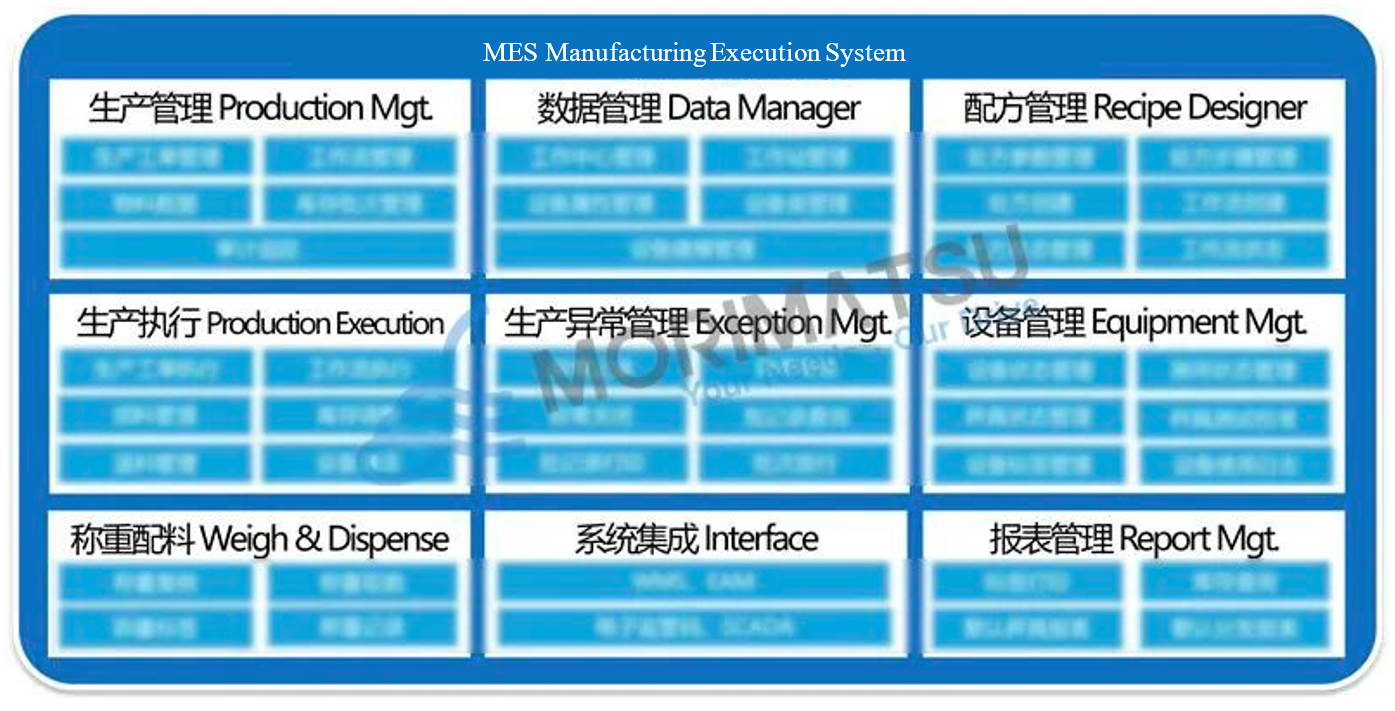
Morimatsu MES System Core Modules
Core Advantages of Morimatsu MES
1.Deep Industry Experience, Precise Pain Point Identification
With a strong understanding of pharmaceutical GMP requirements and manufacturing operations, Morimatsu precisely addresses integration challenges between equipment and processes. Our experienced MES specialists work closely with process experts to ensure efficient collaboration and solutions that are grounded in actual production needs.
2.Proven Platform with End-to-End Delivery Capabilities
Built on partnerships with leading platforms such as Siemens and Rockwell PharmaSuite, Morimatsu MES leverages market-validated core modules. We deliver a fully integrated offering—software, hardware, and implementation—tailored specifically to the needs of pharmaceutical manufacturers.
3.Strong Industry-Specific Customization for Complex Use Cases
Drawing on deep pharmaceutical process knowledge, we optimize MES configurations for a wide range of production scenarios. Standardized data collection interfaces enable efficient integration with diverse pharmaceutical equipment, accelerating deployment while maintaining flexibility.
4.Turnkey Project Delivery with End-to-End Control
Morimatsu provides comprehensive turnkey services spanning modular facilities, automated production lines, and MES systems. With flexible integration of hardware and software, along with professional commissioning and validation support, systems are brought online quickly—delivering measurable results from day one.
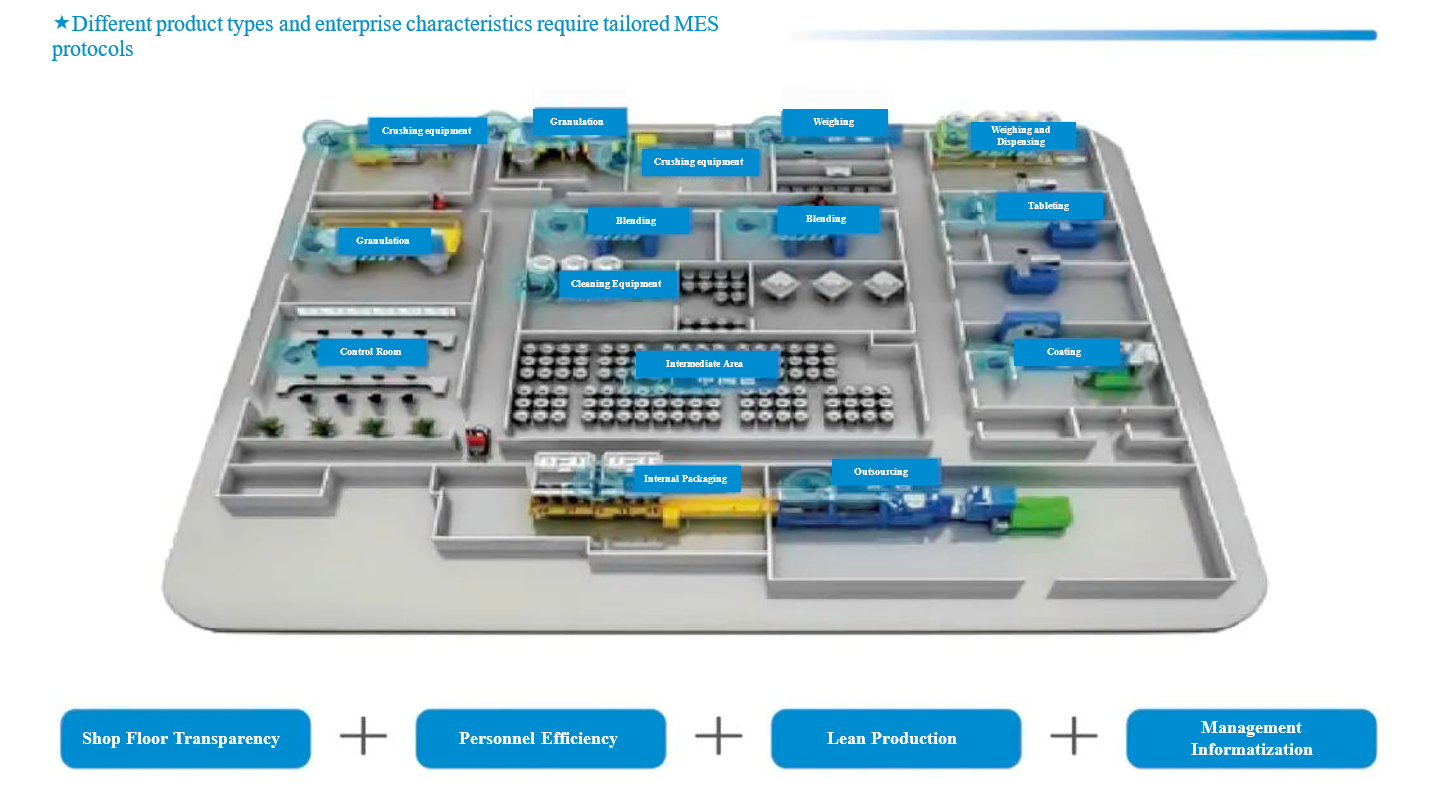
Project Cases
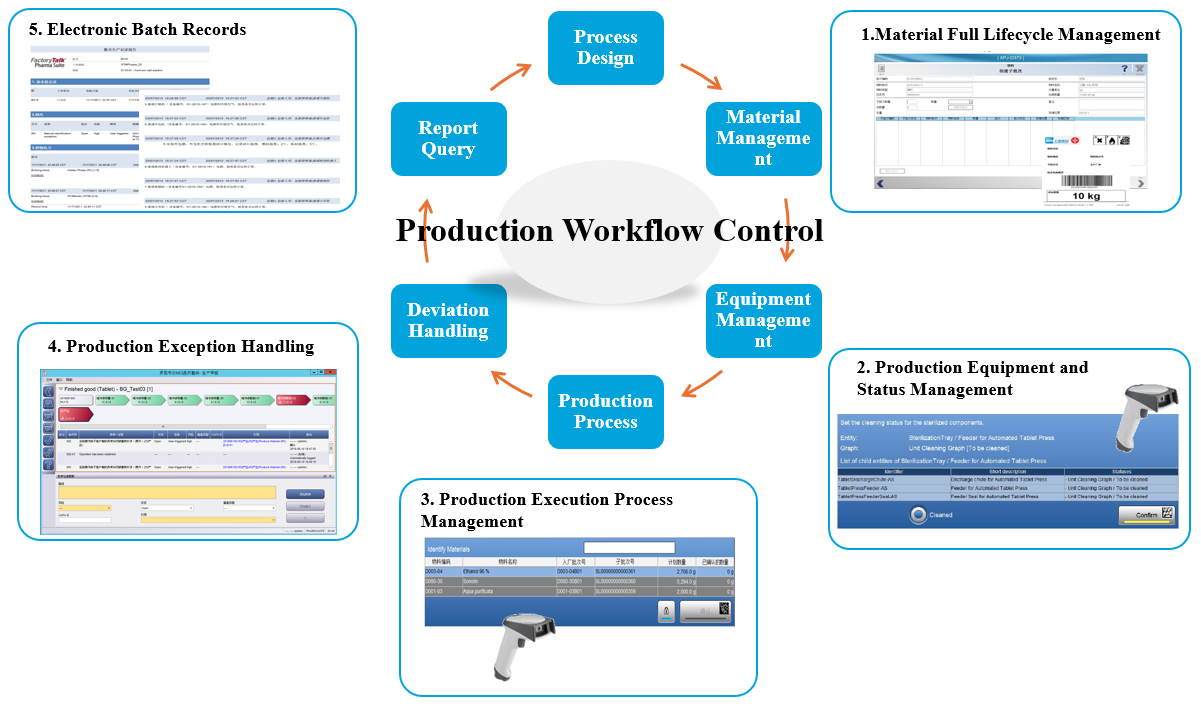
Built on the Rockwell PharmaSuite MES platform, Morimatsu developed a customized digital manufacturing solution for a biopharmaceutical company, directly addressing key challenges such as paper-heavy processes, elevated compliance risk, and limited end-to-end traceability.
1.Core Solution
Paper batch records were fully replaced with Electronic Batch Records (EBR), creating a complete digital workflow supported by automated data capture, electronic signatures, and comprehensive audit trails. The system is fully compliant with GMP requirements and FDA 21 CFR Part 11.
2.Key Implementation Scenarios
Fully Automated Weighing and Batching—when a production order is released, the system automatically converts approved formulations into guided electronic weighing instructions. Operators simply scan material barcodes, while the system verifies material ID, batch number, expiration date, and quality status in real time—preventing material mix-ups and ensuring correct execution at every step.
3.Quantified Results
The implementation delivered a significant reduction in batch record errors and a dramatic improvement in audit preparation efficiency. End-to-end batch traceability was achieved, supported by a complete and reliable data chain from formulation through finished product—providing a strong foundation for lean manufacturing and regulatory compliance.
Built on compliance, designed for efficiency, and driven by end-to-end traceability, the Morimatsu MES solution resolves the limitations of traditional pharmaceutical manufacturing through robust functionality, proven service capabilities, and measurable implementation results. More than a system upgrade, it serves as a strategic enabler of digital transformation—helping pharmaceutical companies strengthen operational excellence, reduce risk, and enhance their core competitiveness while supporting sustainable, high-quality industry growth.
About Morimatsu LifeSciences
Morimatsu LifeSciences is a key business segment of Morimatsu International Holdings Limited (Stock Code: 2155.HK). It comprises Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd., Morimatsu (Suzhou) LifeSciences Co., Ltd., Shanghai Morimatsu Biotechnology Co., Ltd., Shanghai Mori-Biounion Technology Co., Ltd., Shanghai Morisora Technology Co., Ltd., Bioengineering AG, Pharmadule Morimatsu AB, and its affiliated companies.
Morimatsu LifeSciences is dedicated in providing core equipment, process systems, and smart modular facility solutions, and services for the pharmaceutical, biopharmaceutical, medical aesthetics, and fast-moving consumer goods (FMCG) sectors including (cosmetics, food, and health supplements), as well as data centers.
Our team comprises highly experienced professionals with deep expertise in process R&D, engineering design, advanced manufacturing, compliance and validation consulting, production execution, and intelligent operations. With broad experience across diverse industries, we fully understand the unique characteristics and process requirements of various products. This enables us to deliver tailored, end-to-end process solutions from the conceptual design stage, precisely aligned with client’s specific needs.
Morimatsu LifeSciences has established a strong global presence, supported by advanced R&D centers, design hubs, and state-of-the-art manufacturing facilities worldwide. Our well-established service network spans Europe,USA,Asia-Pacific, and emerging markets. We have successfully delivered outstanding, customized solutions to clients in over 40 countries and regions, gaining extensive experience in international project execution.
As a multinational enterprise with core strengths in process technology, modular facility construction, and intelligent manufacturing, Morimatsu LifeSciences is dedicated to meeting the evolving equipment and system needs of our key industries. Through continuous innovation and technological advancement, we are steadily expanding our global footprint, driving our international strategy forward, and delivering Morimatsu’s expertise, reliability, and innovation to the global life sciences and related sectors.