
Search

In the pharmaceutical industry, equipment management is associated with not only production efficiency but essentially with drug quality and GMP compliance.
Traditional equipment management methods often face the following challenges:
Paper-based records are cumbersome with non-standardized signatures, making it difficult to meet GMP compliance requirements
Unplanned downtime causes production disruptions, adversely affecting manufacturing output.
Slow maintenance response times with fault diagnosis relying on empirical experience
Heavy validation workload
Fragmented equipment lifecycle data with poor traceability, manual collection of validation reports, and inefficient lifecycle management
To address these challenges, Morimatsu Digital Team has developed the Enterprise Asset Management System (Mo-EAMaster) by leveraging over 30 years of experience serving global pharmaceutical clients, providing pharmaceutical enterprises with a powerful tool for digital transformation of asset management.
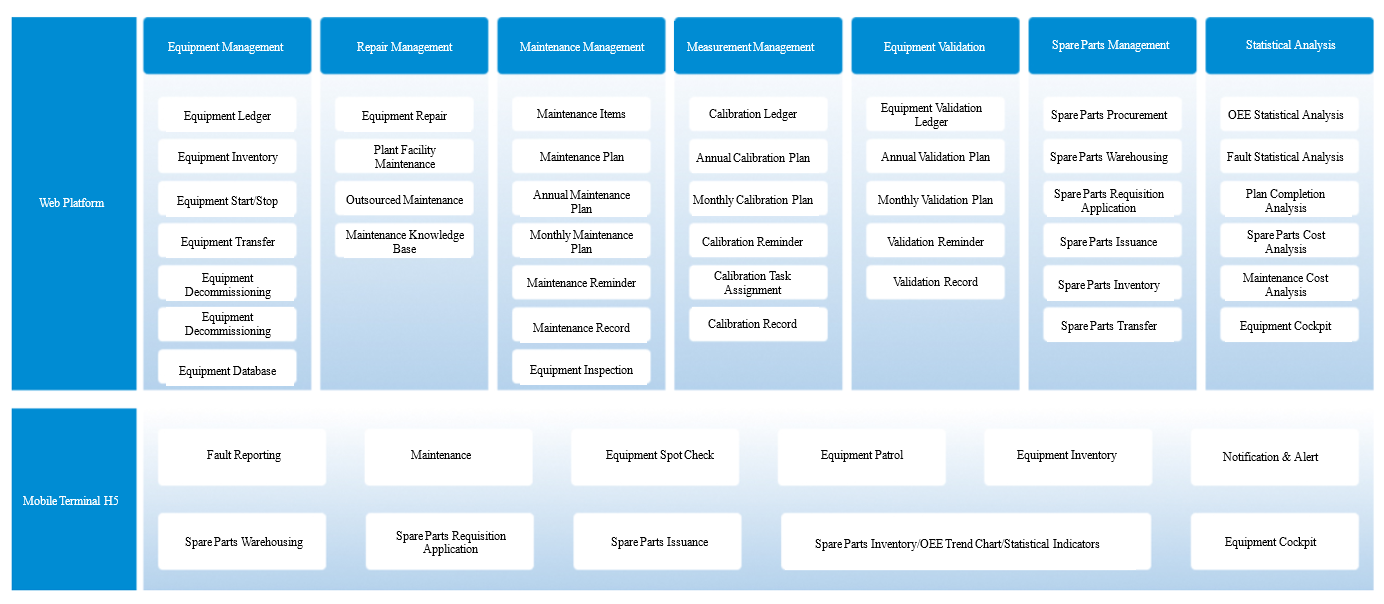
Features of Morimatsu Mo-EAMaster
Morimatsu Mo-EAMaster is an enterprise asset management system specifically designed for the pharmaceutical industry. Based on ISO55000 international standards, GMP and related guidelines, it incorporates Morimatsu's decades of experience in pharmaceutical equipment manufacturing and maintenance, offering pharmaceutical enterprises a full lifecycle digital management solution covering equipment procurement, design, construction, installation, operation and maintenance through to decommissioning.
The system features both PC and mobile terminal functionality. The mobile terminal includes caching capability, allowing normal equipment inspections to continue in cleanroom areas with temporary WiFi or mobile signal outages. All data automatically synchronizes to servers when connectivity is restored.
It supports enterprise private cloud deployment, with centralized deployment enabling multi-site usage while ensuring complete database isolation between sites. Data remains on-premises, delivering dual guarantees for both security and compliance.
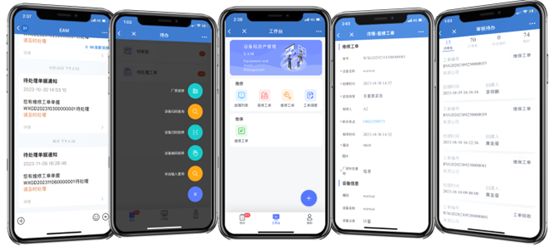
It features comprehensive data isolation and permission management functions, personnel with different access levels can freely customize system metrics relevant to their roles.
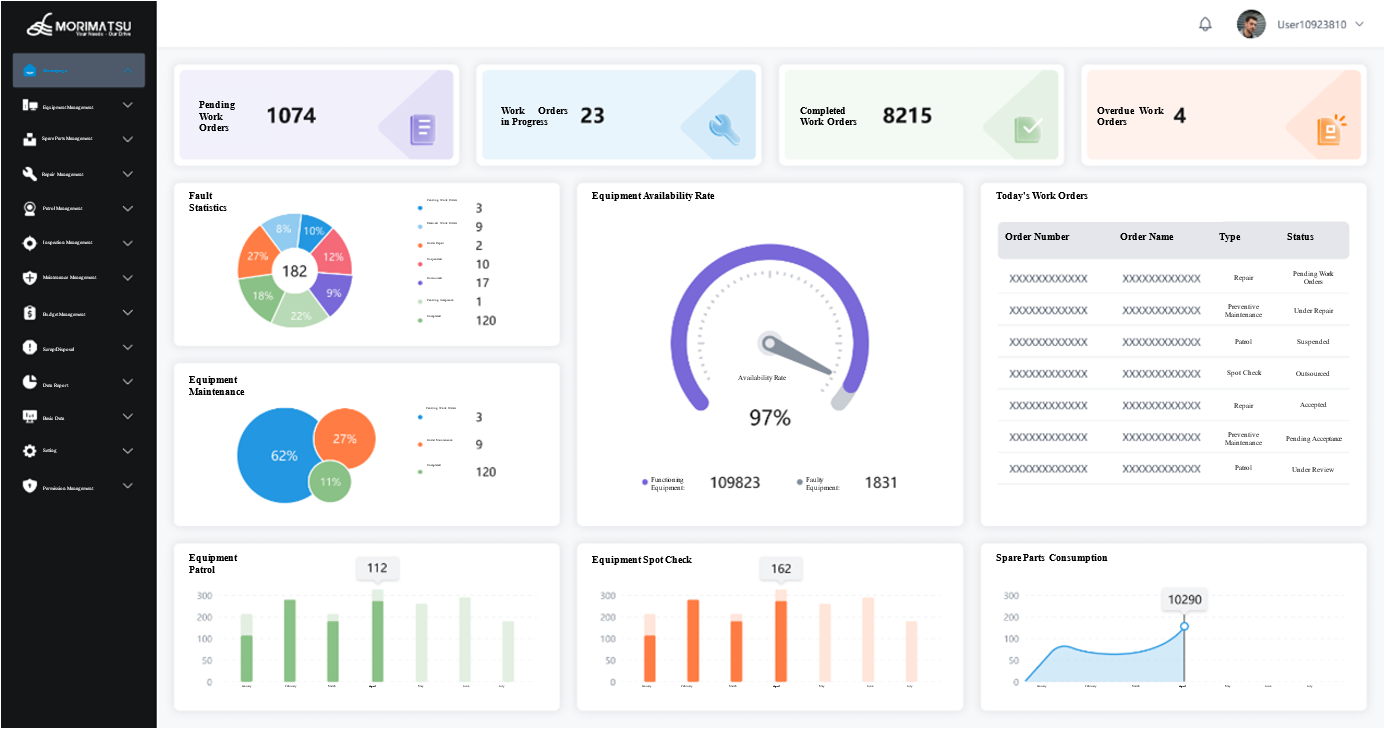
Core Advantages of Morimatsu Mo-EAMaster
1.GMP-Compliant Design
Fully compliant with GAMP5, completing validation for both software and project development
Electronic signatures and permission management ensure data integrity, fully meeting FDA 21 CFR Part 11 requirements
Built-in audit trail functionality
Automated collection of validation records for effortless compliance with various audits
2.Intelligent Approval and Workflow Management
Graphical customizable approval processes
Automatically synchronizes with HR system organizational structures and intelligently routes approvals
Electronic work order circulation replaces paper-based approvals.
3.Equipment Full Lifecycle Management
Provides complete queryable digital archives from procurement to decommissioning.
Seamlessly integrates with Morimatsu's digital delivery platform "Engineering Data Center," supporting viewing of standard format documents (Word, PDF, images, videos) and 3D drawings created by PDMS, SolidWorks, e3D, AutoCAD and other software to review equipment-related design and manufacturing documentation.
Enables one-click retrieval of all documents associated with equipment procurement, design, construction, and installation processes.
4.Automated Validation Management
Automatically generates validation plans (annual/monthly)
Smart alerts for validation cycles
Color-coded validation status for clear progress visibility
5.Predictive Maintenance with AI Assistance
Private AI deployment ensures data security
Intelligent diagnostic recommendations based on maintenance history
Fault prediction to reduce unplanned downtime
Dynamic knowledge base + repair guidance enables even novices to become experts

6.Industry-Specific Functional Modules
Online initiation and execution of equipment impact assessments
Automatic association of critical components with maintenance plans
Currently, over 20 pharmaceutical enterprises have adopted Morimatsu Mo-EAMaster system, achieving:
More than 40% improvement in equipment management efficiency
Over 35% reduction in unplanned downtime
Over 50% reduction in validation and audit preparation time
Over 30% decrease in maintenance costs
Some clients have achieved paperless full lifecycle management of enterprise assets through implementing Morimatsu Mo-EAMaster system, significantly improving management efficiency.
In the future, Morimatsu Mo-EAMaster system will continue to innovate and deeply integrate AI technologies:
1.Intelligent voice-interactive repair guidance
2.Dynamic maintenance plans based on equipment status
3.Big data-driven equipment health prediction
Amid the pharmaceutical industry's comprehensive digital transformation, Morimatsu Mo-EAMaster system breaks through the limitations of traditional tools. It not only possesses powerful functionalities but also incorporates rich industry experience accumulated over years, forming an intelligent solution. We look forward to exploring innovative paths of enterprise asset management with you and advancing steadily in the wave of digital transformation.