
In July 2025, the European Medicines Agency (EMA) and the Pharmaceutical Inspection Co-operation Scheme (PIC/S) introduced the groundbreaking GMP Annex 22, focusing on Artificial Intelligence — the first regulation of its kind for AI applications in the pharmaceutical industry. This new regulation, alongside the updated Annex 11: Computerized Systems, creates a dual-track regulatory framework that formally elevates AI from a simple “technological tool” to a fully GMP-regulated entity.
I. Scope of Application
The scope of application can be summarized as “three permissions and three prohibitions” across the following categories:
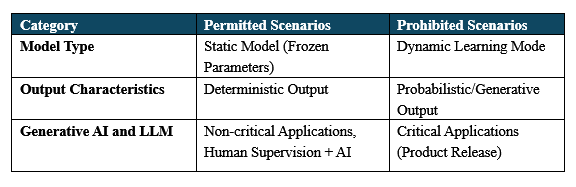
1.Static Models Only
Reason: The continuous learning feature of dynamic models can’t guarantee stable production.
Risk Scenario: If AI models are allowed to learn from new data during production (e.g., automatically adjusting sterilization temperature thresholds), there's a risk of parameter drift, which could be caused by abnormal or contaminated data.
Regulatory Logic: In pharmaceutical manufacturing, reproducibility is critical at every step. Static models are necessary to meet GMP stability requirements and ensure consistent, reliable processes.
2.Deterministic Output is Required
Reason: Pharmaceutical decisions cannot afford to be "close enough."
Regulatory Bottom Line: Drug quality must be entirely predictable and consistent. Any ambiguity could lead to legal challenges, such as disputes over evidence in patient lawsuits.
3.Special Provisions for Generative AI and LLMs
☑ Critical GMP Applications: Strictly prohibited.
☑ Non-Critical Applications: Allowed with human oversight, requiring qualified personnel to ensure the outputs are suitable for their intended use.
II. Core Regulatory Logic
The key requirements in EU GMP Annex 22: Artificial Intelligence reflect a careful balance between the unique demands of the pharmaceutical industry and the risks posed by AI technology. For GMP-critical processes, only AI that is controllable, testable, and verifiable is allowed:
☑ Suitable for critical applications that directly impact patient safety, product quality, or data integrity.
☑ Applicable to static models
☑ Applicable to deterministic output models
☑ Dynamic models prohibited
☑ Generative AI/LLM prohibited
☑ Probabilistic output models prohibited
III. Core regulatory Requirements
The regulations establish a comprehensive compliance framework for the entire lifecycle of AI systems used in the pharmaceutical industry.
1.Development Phase: Triple Lock
01.Data lock
Training data must meet the following requirements:
☑ Legal data sources
☑ Bias control in data
02.Algorithm lock
☑ Algorithms must clearly specify the model’s intended use (e.g., "The model must identify underfilled capsule defects").
☑ Ensure the scientific validity of the algorithm (e.g., avoid bias in the training data).
03.Vendor lock
When using AI models provided by vendors (like purchased AI vision inspection systems), you must obtain additional documentation from the vendor (e.g., training data compliance statements, validation reports) and integrate it into your company’s document management system.
2.Testing Phase: Triple Validation
01.Test plan
Subject Matter Experts (SMEs) should participate in developing the plan.
02.Test data characteristics description
☑ All test documentation must be kept, along with the intended use description, test data characteristics, actual test data, and (if applicable) physical test objects.
☑ This should include a summary of the intended use, predefined metrics, and acceptance criteria, along with references to the test data, test scripts outlining all execution steps, and descriptions of how test metrics are calculated.
03.Deviation report
Any deviations from the test plan, failure to meet acceptance criteria, or missing test data must be documented, investigated, and fully explained.
3.Operation Phase: Real-time Monitoring of the Iron Triangle
01.Performance monitoring records
☑ Regularly monitor model performance to ensure environmental changes (e.g., lighting) don’t affect the outputs.
☑ Periodically check input data for "drift" and set up alerts if it falls outside the model’s expected range.
02.Change control request
Models and their associated systems must be included in change control and configuration management processes before deployment. Adjustments to model parameters are considered major changes and should be reported in the same way as process changes.
03.Manual review records
The manual review process applies to human-machine collaboration, where model outputs are reviewed line by line when needed.
04.Decommissioning Phase: Knowledge Transfer
When AI systems are decommissioned, ensure that historical data remains interpretable (e.g., by preserving feature engineering code) until the relevant product batch records are no longer required.
IV. Core Principles
Regulations explicitly require all AI applications to follow three core principles, which form the foundation for all subsequent operations.
1.Personnel Requirements
To fully understand the intended use and associated risks of AI model applications in GMP environments, all relevant parties should work closely together throughout the algorithm selection, model training, validation, testing, and operational stages. This includes, but is not limited to, process subject matter experts (SMEs), quality assurance (QA), data scientists, IT personnel, and consultants. All personnel must have appropriate qualifications, clearly defined roles, and the necessary access privileges.
☑ Process Subject Matter Experts (SMEs): Responsible for defining the model’s intended use (e.g., "The model must identify underfilled capsule defects") and regularly monitoring performance to assess if environmental factors (e.g., lighting) affect outputs.
☑ Data Scientists: Ensure the scientific integrity of the model's algorithms (e.g., by avoiding bias in the training data).
☑ Quality Assurance (QA): Oversee compliance throughout the entire process (e.g., ensuring test data independence).
☑ IT Personnel: Ensure system stability (e.g., managing access control and audit trails).
2.Documentation Requirements
All documentation related to the activities outlined in this section should be accessible and reviewed by regulated users, whether the model is developed, validated, and tested internally or provided by vendors or service providers.
01.Documentation scope (full lifecycle coverage)
☑ Development Phase: Documentation of algorithm selection rationale, sources of training data, and data cleaning records, along with model parameter configurations.
☑ Testing Phase: Test plans, descriptions of test data characteristics, and deviation reports.
☑ Operation Phase: Records of performance monitoring, change control requests, and manual review documentation.
02.Special requirements for third-party models
When using AI models provided by vendors (like purchased AI vision inspection systems), you must obtain additional documentation from the vendor (e.g., training data compliance statements, validation reports) and integrate it into your company’s document management system.
3.Quality Risk Management
01.Risk assessment matrix
Risk levels are categorized by "Impact Severity" (high/medium/low) and "Probability of Occurrence" (high/medium/low). For example:
☑ High-risk scenarios (e.g., AI models used for sterility testing of sealed products): Require comprehensive, full-process testing with a 50% increase in test data volume.
☑ Low-risk scenarios (e.g., AI models for raw material label recognition): Certain testing steps can be streamlined (e.g., reducing the number of subgroups tested).
02.Risk review frequency
If model performance drift occurs (e.g., a decline in accuracy), an immediate risk reassessment must be initiated.
Through collaborative efforts, the regulatory framework for "AI + Pharmaceuticals" is evolving, paving the way for global drug production and regulation to enter a new era of intelligence and coordination.
Next, we will explore the key requirements for implementing AI.
About Morimatsu LifeSciences
Morimatsu LifeSciences is a key business segment of Morimatsu International Holdings Limited (Stock Code: 2155.HK). It comprises Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd., Morimatsu (Suzhou) LifeSciences Co., Ltd., Shanghai Morimatsu Biotechnology Co., Ltd., Shanghai Mori-Biounion Technology Co., Ltd., Shanghai Morisora Technology Co., Ltd., Bioengineering AG, Pharmadule Morimatsu AB, and its affiliated companies.
Morimatsu LifeSciences is dedicated in providing core equipment, process systems, and smart modular facility solutions, and services for the pharmaceutical, biopharmaceutical, medical aesthetics, and fast-moving consumer goods (FMCG) sectors including (cosmetics, food, and health supplements), as well as data centers.
Our team comprises highly experienced professionals with deep expertise in process R&D, engineering design, advanced manufacturing, compliance and validation consulting, production execution, and intelligent operations. With broad experience across diverse industries, we fully understand the unique characteristics and process requirements of various products. This enables us to deliver tailored, end-to-end process solutions from the conceptual design stage, precisely aligned with client’s specific needs.
Morimatsu LifeSciences has established a strong global presence, supported by advanced R&D centers, design hubs, and state-of-the-art manufacturing facilities worldwide. Our well-established service network spans Europe,USA,Asia-Pacific, and emerging markets. We have successfully delivered outstanding, customized solutions to clients in over 40 countries and regions, gaining extensive experience in international project execution.
As a multinational enterprise with core strengths in process technology, modular facility construction, and intelligent manufacturing, Morimatsu LifeSciences is dedicated to meeting the evolving equipment and system needs of our key industries. Through continuous innovation and technological advancement, we are steadily expanding our global footprint, driving our international strategy forward, and delivering Morimatsu’s expertise, reliability, and innovation to the global life sciences and related sectors.